Looking ahead
Emerging treatments for LGSOC
Recent advances in the biological understanding of the mechanism of disease have opened up new targets and pathways for research to explore.1 Several investigational trials are now underway, each with the goal of establishing effective therapies for patients living with LGSOC.1,2
Innovative approaches under investigation
There is a need for more treatment options for patients diagnosed with LGSOC2
~70% of LGSOC patients have RAS/MAPK pathway-associated mutations.3-6
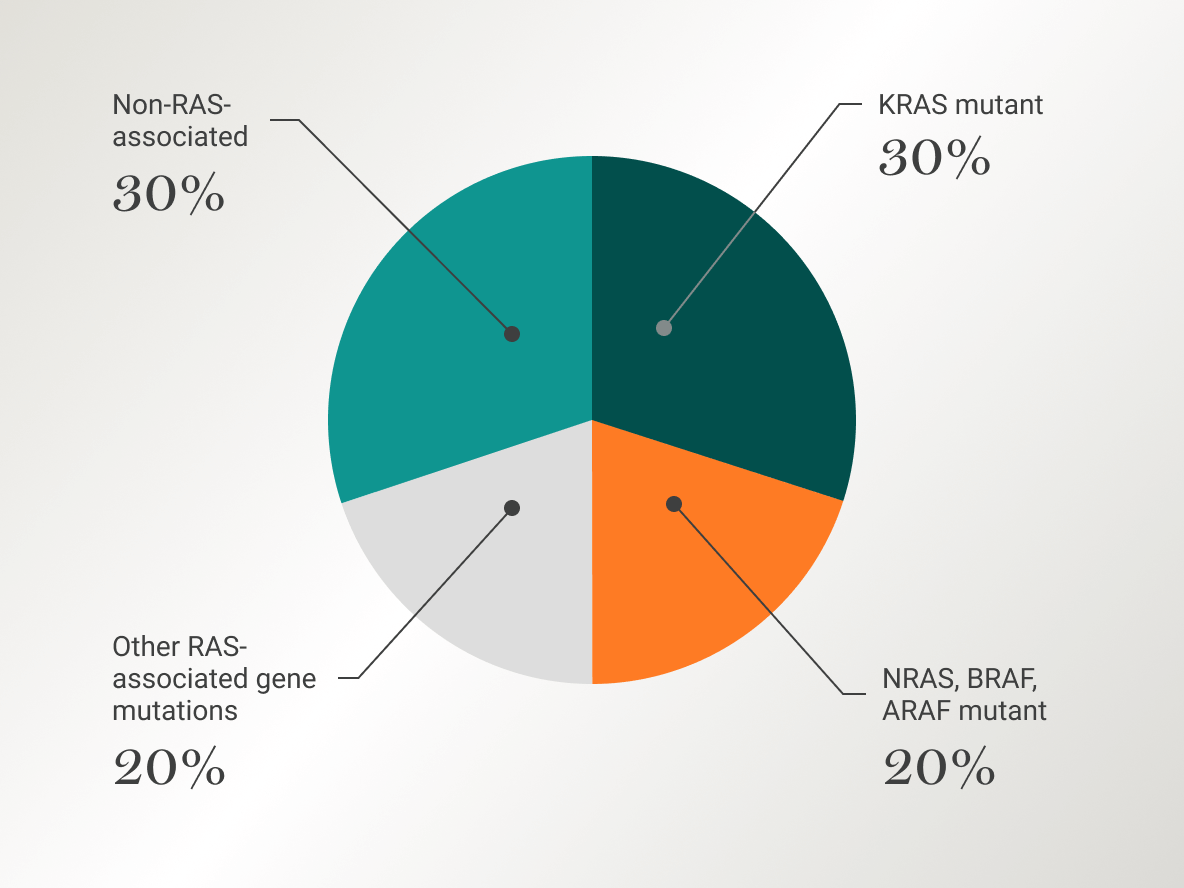
RAS/MAPK alterations include genes such as KRAS, BRAF, NRAS, and EIF1AX3
The rat sarcoma virus/mitogen-activated protein kinase (RAS/MAPK) pathway plays a crucial role in controlling gene expression, cellular growth, and survival mechanisms. Anomalies in the RAS/MAPK pathway have been strongly associated with the onset and advancement of various cancer forms, including LGSOC. The strategy of targeting and inhibiting the RAS/MAPK pathway is at the forefront of current research efforts. Despite this, the observed efficacy has been restricted, underscoring the continued need for dedicated research in this area.3,4
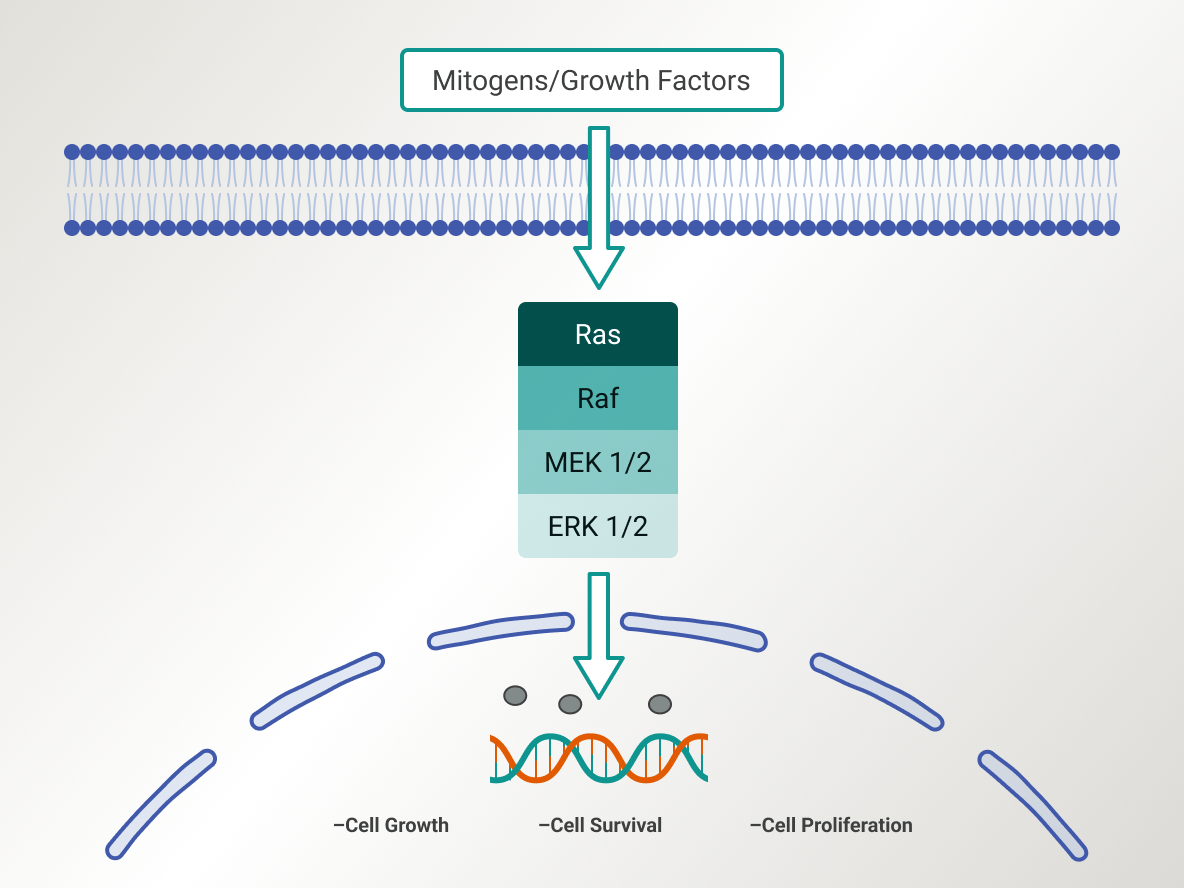
Simplified schematic of the RAS/MAPK pathway1
A key signaling pathway that regulates a wide variety of cellular processes including proliferation, apoptosis, and stress responses, the RAS/MAPK pathway plays a crucial role in LGSOC and serves as an important target for therapies such as MEK and RAF inhibitors.1
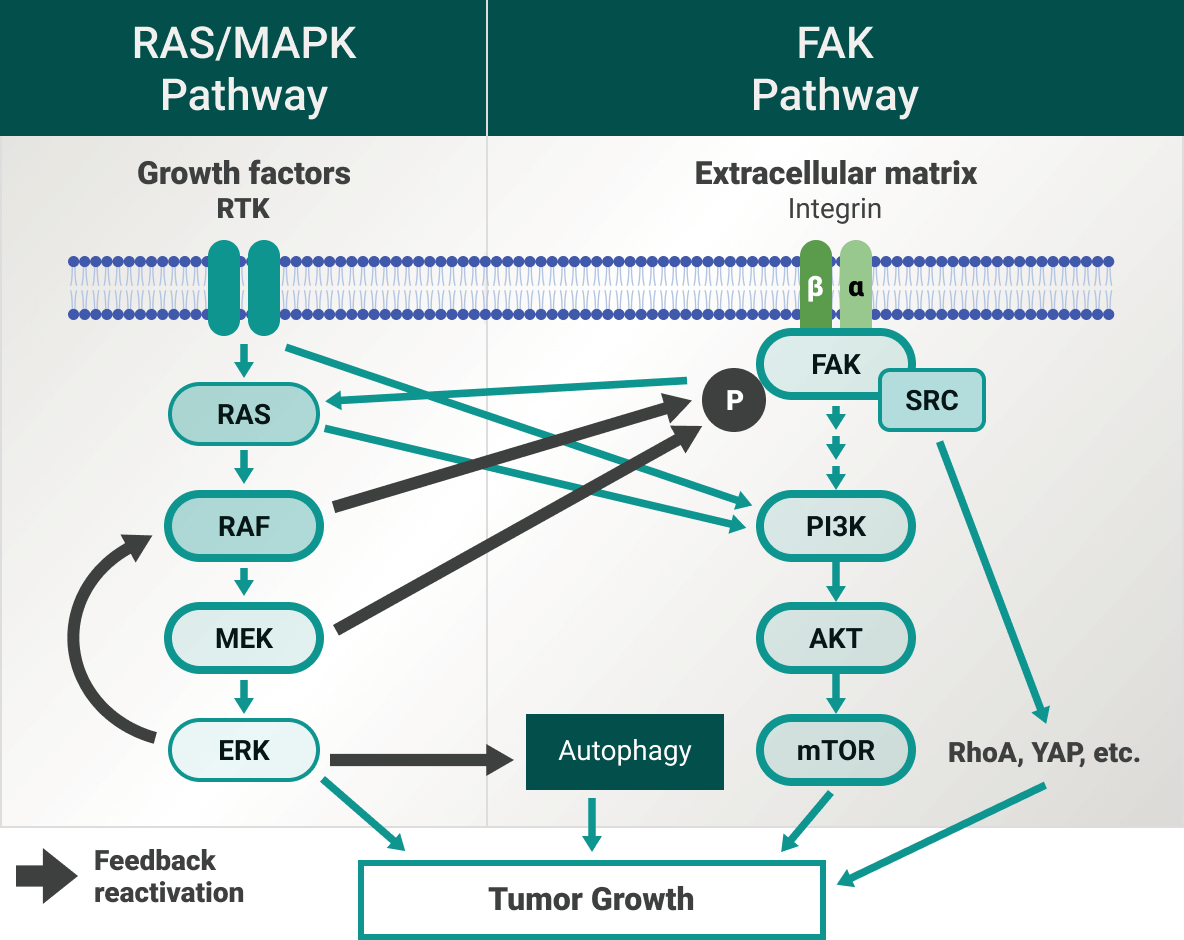
Overcoming the challenge of multiple resistance mechanisms
RAS is one of the best-known signaling proteins implicated in the initiation and progression of cancer. While the RAS/MAPK pathway is promising, it has been observed that inhibiting only a single target of the RAS/MAPK pathway may cause reactivation of another oncogene on the same pathway.7
Single-target therapies (eg, MEK-only inhibitors) are associated with resistance and may not be the best avenue to slowing tumor growth, and finding tolerable combination regimens with MEK-only inhibitors has been challenging.7
Focal adhesion kinase (FAK) functions as a master regulator of drug resistance.8 It is at the intersection of various signaling pathways promoting cancer growth and metastasis, and there are currently over 15 clinical trials to better understand the FAK pathway and its implications in multiple cancer types.9-12 Blocking the RAS/MAPK pathway may trigger activation of other pathways, such as FAK, as an adaptive resistance mechanism.7,9,13-17
Ongoing studies of therapies that inhibit multiple targets in the RAS/MAPK pathway may guide future treatment options.3
Sign up to receive the latest LGSOC consensus publication.
Other important targets in LGSOC
Recent advances in the biological understanding of the mechanism of disease have opened up new targets and pathways for research to explore.1 In addition to KRAS and BRAF, several other genetic alterations are under investigation for their potential involvement in the pathogenesis of LGSOC. Some of these include CDKN2A/2B deletion, CDK 4/6, MEK, NRAS, ERBB2, ERBB3, PIK3CA alterations, neurofibromin 1 (NF1), and chromosome 1p36 deletion.2,3,18,19
Snapshot of clinical trials for LGSOC
Updated as of 8/6/24
| Investigational Drug | MoA | Phase | Trial | Treatment Setting |
|---|---|---|---|---|
| Trametinib + navitoclax 18 | MEK inhibitor + BCL2 inhibitor | Ib/II | NCT02079740 | Advanced solid tumors that carry KRAS or NRAS mutations, including ovarian tumors |
| Abemaciclib + fulvestrant18 | CDK4/6 inhibitor | II | NCT03531645 | Neoadjuvant therapy in women with unresectable, untreated stage III-IV LGSOC |
| Avutometinib vs avutometinib + defactinib20,21 | Dual RAF/MEK inhibitor + FAK inhibitor | II | RAMP 201 NCT04625270 | Recurrent LGSOC with and without KRAS mutation |
| Biomarker-driven therapies22 | Multiple MOAs based on biomarker | II | BOUQUET NCT04931342 | Persistent or recurrent rare epithelial ovarian, fallopian tube, or primary peritoneal tumors |
| Onapristone ± anastrozole23 | PR inhibitor | II | NCT03909152 | PR-positive gynecologic cancers including recurrent LGSOC |
| Pembrolizumab + chemotherapy18 | ICI | II | PERCEPTION NCT04575961 | Platinum-sensitive recurrent LGSOC |
| Regorafenib + fulvestrant18 | Antiangiogenic agent | II | NCT05113368 | Recurrent LGSOC |
| Ribociclib + letrozole18 | CDK4/6 aromatase inhibitor | II | GOG 3026 NCT03673124 | Recurrent LGSOC |
| Palbociclib + binimetinib24 | Kinase inhibitors | II | ComboMATCH Treatment Trial NCT05554367 | RAS-mutated cancers; previously treated with a MEK inhibitor |
| Abemaciclib + letrozole25 | Aromatase inhibitor + CDK4/6 inhibitor | II | ALEPRO NCT05872204 | Recurrent estrogen receptor-positive rare ovarian cancer |
| Avutometinib + defactinib26 | Kinase inhibitors | III | RAMP 301 NCT06072781 | Recurrent LGSOC |
| Letrozole18 | Aromatase inhibitor | III | MATAO NCT04111978 | ER-positive HGSC, LGSOC, or endometrial ovarian tumors, stages II-IV whose cancer has not progressed on platinum-based chemotherapy |
| Letrozole + paclitaxel and carboplatin18 | Aromatase inhibitor | III | NRG-GY-019 NCT04095364 | Stage II-IV LGSOC, fallopian tube, or primary peritoneal cancer |
The role of clinical trials in treatment progress

Unique targets
In recent years, new insights into the clinical and molecular features of LGSOC have led to prospective clinical trials that focus on improving low-grade serous ovarian cancer management and treatment.1,2
Targeting unique characteristics of LGSOC and learning from prognostic markers may help tailor sequencing and treatment options.1 Current clinical trials in LGSOC focus on:
- Optimizing front-line strategies in the neoadjuvant and adjuvant settings1
- Maximizing the effectiveness of recurrence strategies with endocrine therapy and RAS/MAPK pathway-targeting drugs1

Trials in progress
Receive the latest information about LGSOC.
References
- Grisham RN, Manning-Geist BL, Chui MH. The highs and lows of serous ovarian cancer. Cancer. 2023;129(17):2613-2620.
- Zwimpfer TA, Tal O, Geissler F, et al. Low grade serous ovarian cancer – a rare disease with increasing therapeutic options. Cancer Treat Rev. 2023;112:102497.
- Manning-Geist B, Gordhandas S, Liu YL, et al. MAPK pathway genetic alterations are associated with prolonged overall survival in low-grade serous ovarian carcinoma. Clin Cancer Res. 2022;28(20):4456-4465.
- Gershenson DM, Cobb LP, Westin SN, et al. Contemporary primary treatment of women with stage II-IV low-grade serous ovarian/peritoneal cancer (LGSOC): determinants of relapse and disease-free survival. Gynecol Oncol. 2022;167(2):139-145.
- ElNaggar A, Robins D, Baca Y, et al. Genomic profiling in low grade serous ovarian cancer: identification of novel markers for disease diagnosis and therapy. Gynecol Oncol. 2022;167(2):306-313.
- Thomson JP, Hollis RL, van Baal J, et al. Whole exome sequencing of low grade serous ovarian carcinoma identifies genomic events associated with clinical outcome. Gynecol Oncol. 2023;174:157-166.
- Kun E, Tsang YTM, Ng CW, et al. MEK inhibitor resistance mechanisms and recent developments in combination trials. Cancer Treat Rev. 2021;92:102137.
- Schlaepfer D, Ojalill M, Stupack D. Focal adhesion kinase signaling–tumor vulnerabilities and clinical opportunities. Journal of Cell Science. 2024;137:1-14.
- Dawson JC, Serrels A, Stupack DG, et al. Targeting FAK in anticancer combination therapies. Nat Rev Cancer. 2021;21(5):313-324.
- Turapov T, Parkman GL, Stanley KA, et al. Characterizing focal adhesion kinase and MAPK crosstalk in NRAS and BRAF mutant melanoma. Abstract presented at: 19th International Congress of the Society for Melanoma Research. October 17-20, 2022; Edinburgh, Scotland.
- Lubrano S, Faraji F, Arang N, et al. FAK inhibition combined with the RAF/MEK clamp avutometinib overcomes resistance to BRAF/MEK inhibitors and immune checkpoint blockage in BRAFV600E mutant cutaneous melanoma. Paper presented at: 20th International Congress of the Society for Melanoma Research. November 6-9, 2023; Philadelphia, PA.
- Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14(9):598-610.
- Alessi DR, Cuenda A, Cohen P, et al. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270(46):27489-27494.
- Moore AR, Rosenberg SC, McCormick F, et al. RAS-targeted therapies: is the undruggable drugged? Nat Rev Drug Discov. 2020;19(8):533-552.
- Lito P, Saborowski A, Yue J, et al. Disruption of CRAF-mediated MEK activation is required for effective MEK inhibition in KRAS mutant tumors. Cancer Cell. 2014;25(5):697-710.
- Shinde R, Terbuch A, Little M, et al. Phase I study of the combination of a RAF-MEK inhibitor CH5126766 and FAK inhibitor defactinib in an intermittent dosing schedule with expansions in KRAS mutant cancers. Abstract CT143 presented at: Annual Meeting of the American Association for Cancer Research; Apr 27-28 and Jun 22-24, 2020; Philadelphia, PA.
- Chen G, Gao C, Gao X, et al. Wnt/β-catenin pathway activation mediates adaptive resistance to BRAF inhibition in colorectal cancer. Mol Cancer Ther. 2018;17(4):806-813.
- Grisham RN, Slomovitz BM, Andrews N, et al. Low-grade serous ovarian cancer: expert consensus report on the state of the science. Int J Gynecol Cancer. 2023;33(9):1331-1344.
- Van Nieuwenhuysen E, Busschaert P, Laenen A, et al. Loss of 1p36.33 frequent in low-grade serous ovarian cancer. Neoplasia. 2019;21(6):582-590
- ClinicalTrials.gov. A study of avutometinib (VS-6766) v. Avutometinib (VS-6766) + Defactinib in recurrent low-grade serous ovarian cancer with and without a KRAS mutation (RAMP 201). Accessed July 9, 2024. https://www.clinicaltrials.gov/study/NCT04625270
- Banerjee SN, Ring KL, Nieuwenhuysen EV, et al. Initial efficacy and safety results from ENGOT-ov60/GOG-3052/RAMP 201: a phase 2 study of avutometinib (VS-6766) ± defactinib in recurrent low-grade serous ovarian cancer (LGSOC). J Clin Oncol. 2023;41(suppl 16):5515.
- ClinicalTrials.gov. A study evaluating the efficacy and safety of biomarker-driven therapies in patients with persistent or recurrent rare epithelial ovarian tumors (BOUQUET) Accessed September 28, 2024. https://clinicaltrials.gov/study/NCT04931342
- ClinicalTrials.gov. A study of onapristone ER alone or in combination with anastrozole in gynecologic cancers that respond to progesterone. Accessed July 9, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT03909152
- ClinicalTrials.gov. Palbociclib and binimetinib in RAS-mutant cancers, a ComboMATCH Treatment Trial. Accessed July 9, 2024. https://clinicaltrials.gov/study/NCT05554367
- ClinicalTrials.gov. Abemaciclib and Letrozole in patients with estrogen receptor-positive rare ovarian cancer (ALEPRO). Accessed July 9, 2024. https://clinicaltrials.gov/study/NCT05872204
- ClinicalTrials.gov. A study of avutometinib + defactinib (VS-6063) in recurrent low-grade serous ovarian cancer (RAMP 301). Accessed July 9, 2024. https://clinicaltrials.gov/study/NCT06072781