Low-grade serous ovarian cancer (LGSOC)—a distinct ovarian cancer with limited treatment options
Managing patients with LGSOC
LGSOC is molecularly and histopathologically distinct and clinically different: it behaves differently, responds differently, and deserves different treatment.1,2 While once thought to be on a continuum with high-grade serous ovarian cancer (HGSOC), LGSOC is now understood to be a separate ovarian cancer with characteristics distinct from HGSOC, and management depends on the differentiation of LGSOC from HGSOC.3 However, there are no FDA-approved treatments specifically for LGSOC.4
A significant burden for patients
Quality of life challenges
LGSOC affects a younger patient population and can disproportionately impact health, fertility, and long-term quality of life.2,5,6
Patients overwhelmingly report a negative impact of LGSOC on core aspects of their life.6
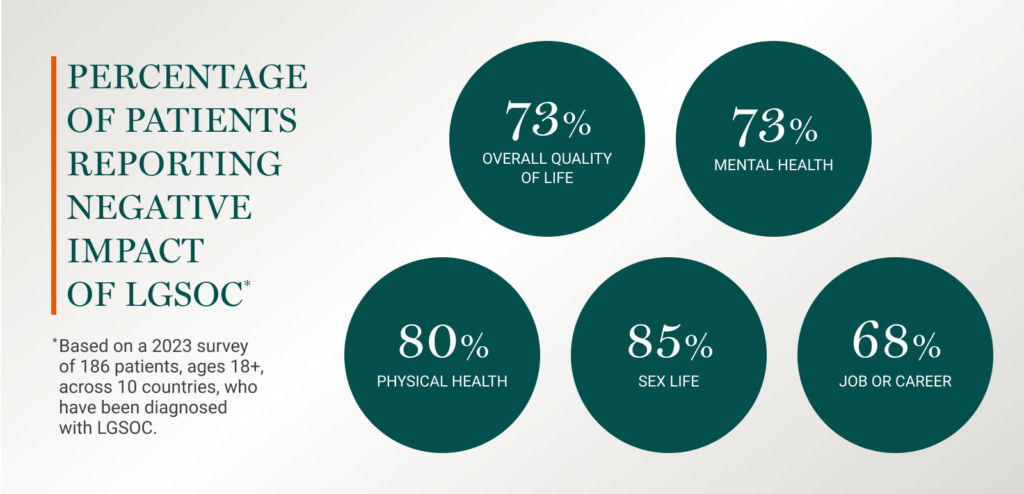
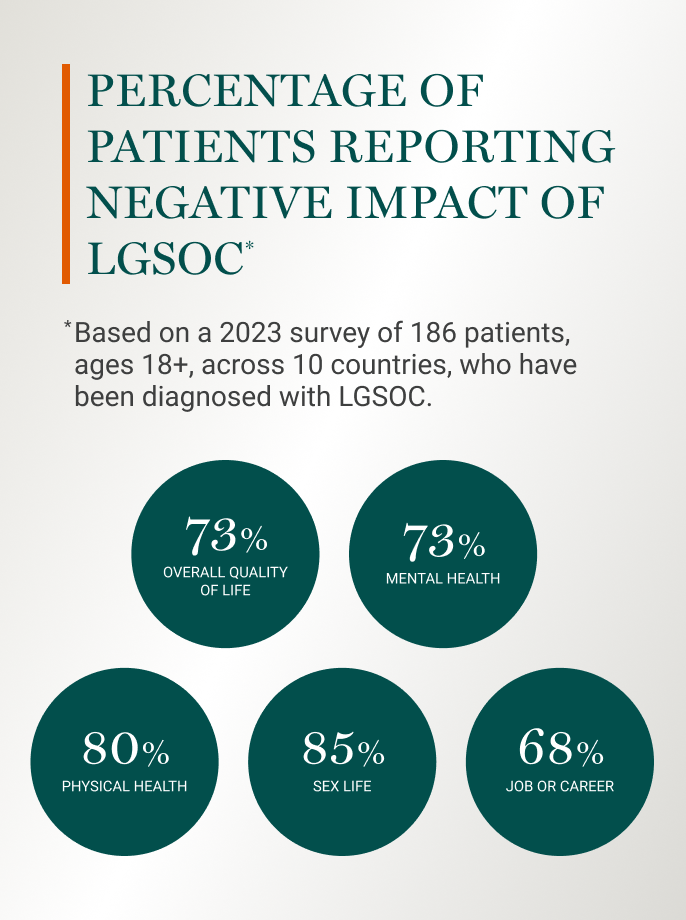
The biggest emotional burden for most patients (~70%) was living every day not knowing if their cancer would return.6
Clearly, there is a need for more effective and tolerable treatment options for patients diagnosed with LGSOC.7-10
Coping with recurrence
Amanda, a patient with LGSOC, discusses the challenges of living with recurrent disease.
Current LGSOC treatment challenges
Patients with LGSOC have inadequate treatment options with limited efficacy, concerning toxicities, and high discontinuation rates. Current treatments (no treatments are currently approved specifically for LGSOC) offer poor to moderate response (0-26%).7-23 The result is a median overall survival, including younger patients, of less than 11 years.24
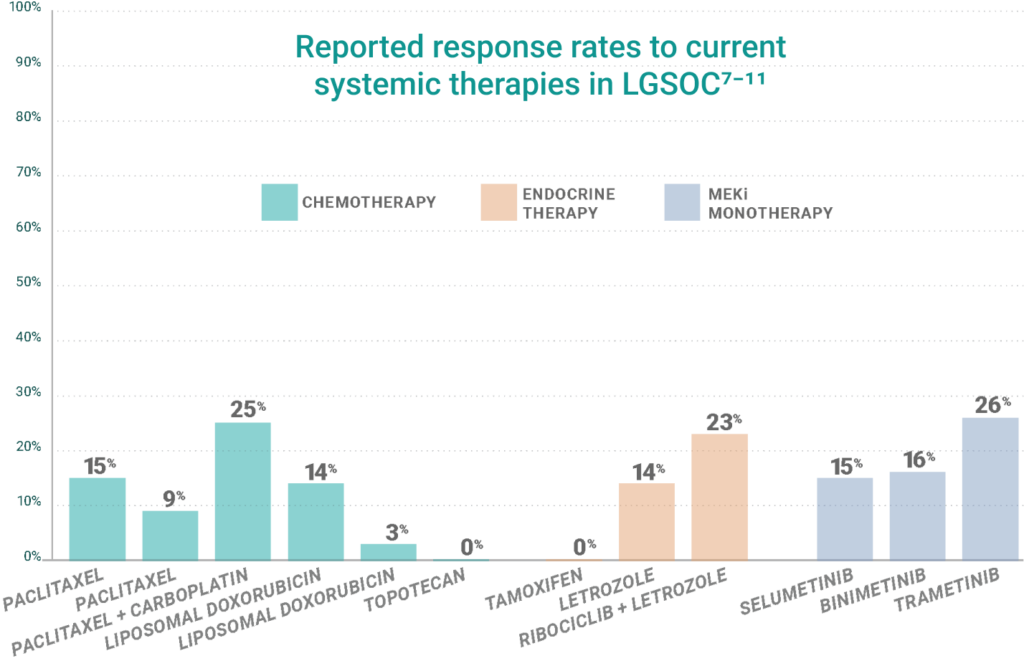
Some agents listed multiple times due to variance in study results.
Chemotherapy for low-grade serous ovarian cancer
LGSOC is less sensitive to chemotherapy compared to the higher response rate of HGSOC in recurrent settings.25
Studies consistently show the lower responsiveness of LGSOC to chemotherapy compared to HGSOC.26 Further, an analysis of patients with LGSOC from the National Cancer Database demonstrated that there was no difference in overall survival between patients that received adjuvant chemotherapy vs. patients that did not—demonstrating that this strategy may not be associated with an overall survival benefit following primary cytoreductive surgery.27
Endocrine therapy for LGSOC
- Patients who are receiving AIs should be counseled regarding musculoskeletal side effects17:
- These may be managed through exercise; acupuncture; application of heat or cold; use of a nonsteroidal anti-inflammatory drug (NSAID) or other analgesics, a diuretic, duloxetine, or omega-3 fatty acids; conversion to an alternative AI; and an AI holiday17,31-35
- Patients should be monitored for bone mineral density and, for appropriate patients, treatment should be initiated36
MEK-only inhibitors
Efficacy of MEK-only inhibitors is limited (15-26% response rates) with high discontinuation rates (over 30% for trametinib and binimetinib).7,9,10 It is important to monitor patients for MEKi-related toxicities and address side effects as they arise.17,18
MEK-only inhibitor-specific toxicities may include:
- Cutaneous skin reactions, diarrhea, peripheral edema, cardiac toxicity, ocular toxicity, and interstitial lung disease10,17
National Comprehensive Cancer Network® (NCCN®)
NCCN Guidelines treatment recommendations
NCCN-recommended treatment for LGSOC stages IA-IC
Cytoreductive surgery is the primary treatment for all epithelial ovarian cancers37
Fertility-sparing surgery is an option in stage IA-C1 LGSOC. Oocyte retrieval from an unaffected ovary is an option in the surgical management of LGSOC.17,37
Adjuvant treatment options
Stage IA-IB37
Observe
(Consider surgical setting and resection of residual disease if not done previously)
Stage IC37
- Observe (category 2B)a
- Systemic chemotherapyb
- Followed by observation, maintenance letrozole, or other hormonal therapy (2B)c
- Hormonal therapy (category 2B)b
Preferred Regimens for Stage 1C
- Paclitaxel/carboplatin q3weeksd,e ± maintenance letrozole,f or other hormonal therapy (2B)c
- Hormone therapy (aromatase inhibitors: letrozole,f anastrozole, exemestane) (2B)
Other recommended regimens
- Carboplatin/liposomal doxorubicin ± maintenance letrozolef or other hormonal therapy (2B)c
- Docetaxel/carboplatin ± maintenance letrozolef or other hormonal therapy (2B)c
- Hormone therapy (leuprolide acetate, goserelin acetate, tamoxifen,g fulvestrant) (2B)
Useful in certain circumstances
- Paclitaxel/cisplatin
Note: All recommendations are category 2A unless otherwise indicated.
aIf not previously done, consider surgical staging and resection of residual disease.
bSee Principles of Systemic Therapy (OV-C) and Management of Drug Reactions (OV-D).
cOther hormonal therapy options include: aromatase inhibitors (ie, anastrozole, exemestane), leuprolide acetate, goserelin acetate, or tamoxifen.
dAlbumin-bound paclitaxel may be substituted for those experiencing a hypersensitivity reaction to paclitaxel. However, albumin-bound paclitaxel will not overcome infusion reactions in all patients.
eIndividuals >70 years of age and those with comorbidities may be intolerant to the combination chemotherapy regimens recommended in these NCCN Guidelines. Based on clinical judgment and expected tolerance to therapies, alternate dosing (OV-C, 7 of 12) may be appropriate for these individuals with epithelial ovarian cancer (including carcinosarcoma, clear cell, mucinous, and low-grade serous). Algorithms have been developed for predicting chemotherapy toxicity.
fFor low-grade serous, maintenance letrozole is a category 2A recommendation.
gTamoxifen is not recommended for low-grade serous carcinoma.
Source: Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer V.3.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed July 17, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
NCCN-recommended treatment for LGSOC Stages II-IV37
Cytoreductive surgery is the primary treatment for all epithelial ovarian cancers37
Adjuvant treatment options
Stage II-IV37
- Systemic chemotherapyh
- Followed by maintenance letrozole (2A), or other hormonal therapy (2B)i
- Hormonal therapy (category 2B)h
Preferred regimens
- Paclitaxel/carboplatin q3weeksj,k ± maintenance letrozolel or other hormonal therapy (2B),i
- Paclitaxel/carboplatin/bevacizumab + maintenance bevacizumabj,m
- Hormone therapy (aromatase inhibitors: anastrozole, letrozole, exomestane) (2B)
Other recommended regimens
- Paclitaxel weekly/carboplatin weeklyj,k,n
- Docetaxel/carboplatin ± maintenance letrozolel or other hormone therapy (2B)2,i
- Carboplatin/liposomal doxorubicin ± maintenance letrozolel or other hormonal therapy (2B)i
- Paclitaxel weekly/carboplatin q3weeksj
- Docetaxel/carboplatin/bevacizumab + maintenance bevacizumabm
- Hormone therapy (leuprolide acetate, goserelin acetate, tamoxifen, fulvestant) (2B)
Useful in certain circumstances
- Paclitaxel/crisplatin
- Docetaxel/oxaliplatin/bevacizumab + maintenance bevacizumab (2B)m
Note: All recommendations are category 2A unless otherwise indicated.
hSee Principles of Systemic Therapy (OV-C) and Management of Drug Reactions (OV-D).
iOther hormonal therapy options include: aromatase inhibitors (ie, anastrozole, exemestane), leuprolide acetate, goserelin acetate, or tamoxifen.
jAlbumin-bound paclitaxel may be substituted for those experiencing a hypersensitivity reaction to paclitaxel. However, albumin-bound paclitaxel will not overcome infusion reactions in all patients.
kIndividuals >70 years of age and those with comorbidities may be intolerant to the combination chemotherapy regimens recommended in these NCCN Guidelines. Based on clinical judgment and expected tolerance to therapies, alternate dosing (OV-C, 7 of 12) may be appropriate for these individuals with epithelial ovarian cancer (including carcinosarcoma, clear cell, mucinous, and low-grade serous). Algorithms have been developed for predicting chemotherapy toxicity. See the NCCN Guidelines for Older Adult Oncology.
lFor low-grade serous, maintenance letrozole is a category 2A recommendation.
mAn FDA-approved biosimilar is an appropriate substitute for bevacizumab.
nRegimen may be considered for those with poor performance status.
Monitoring and follow-up for recurrence37
- Visits every 2 to 4 months for 2 years, 3 to 6 months for 3 years, annually after 5 years
- Physical exam, including pelvic exam as clinically indicated
- Tumor molecular testing, if not previously donep
- Refer for genetic risk evaluation, if not previously doneq
- Chest/abdominal/pelvic CT, MRI, PET/CT, or PET (skull base to mid-thigh) as clinically indicatedr
- CBC and chemistry profile as indicated
- CA-125s or other tumor markers if initially elevated
- Long-term wellness care
pValidated molecular testing should be performed in a CLIA-approved facility using the most recent available tumor tissue. Tumor molecular analysis is recommended to include, at a minimum, tests to identify potential benefit from targeted therapeutics that have tumor-specific or tumor-agnostic benefit including, but not limited to, HER2 status (by IHC), BRCA1/2, HRD status, MSI, MMR, TMB, BRAF, FRα (FOLR1), RET, and NTRK if prior testing did not include these markers. More comprehensive testing may be particularly important in LCOC with limited approved therapeutic options (OV-B).
qSee NCCN Guidelines for Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic and NCCN Guidelines for Genetic/Familial High-Risk Assessment: Colorectal.
rCT is preferred with oral and IV contrast (unless contraindicated) and rectal contrast as needed.
sThere are data regarding the utility of CA-125 for monitoring of ovarian cancer after completion of primary therapy. See The Society of Gynecologic Oncology (SGO) position statement and Discussion.
Managing recurrent disease
Treatment options for recurrent low-grade serous ovarian cancer are limited and include secondary cytoreduction, clinical trials, chemotherapy, endocrine therapy, or RAF- and MEK-targeted therapies.37-39 Recent expert consensus is that there is no defined standard of care treatment for recurrent LGSOC.17 Though there are no FDA-approved treatments specifically for LGSOC,4 NCCN does recommend the following:
NCCN recurrence therapy recommendations
Recurrence therapy options17,37
- Secondary cytoreductive surgery
- Trametinibt
- Binimetinib (category 2B)t
- Dabrafenib + trametinib (for BRAF V600E positive tumors)t
- Hormonal therapy
- Use of an aromatase inhibitor (ie, letrozole, anastrozole, exemestane) is preferred if not used previously
- Use fulvestrant, leuprolide acetate or goserelin acetate if an aromatase inhibitor was given previously
- Chemotherapy if not previously used
- Other systemic therapyt,u
- Observation
No standard sequencing of drugs for recurrent disease; individual patient factors will play a role in decision-making37
Note: All recommendations are category 2A unless otherwise indicated.
There are no FDA-approved drugs or treatments specifically for LGSOC.4
Clinical trials and advances in treatment options in
Low-grade serous ovarian cancer
Prospective clinical trials are pivotal in the search for better low-grade serous ovarian cancer management and treatment.40 Patients need guidance finding clinical trials, effective therapies, and a community for socio-emotional support.6
References
- Gershenson DM. Low-grade serous carcinoma of the ovary or peritoneum. Ann Oncol. 2016;27(Suppl 1):i45-i49.
- Slomovitz B, Gourley C, Carey MS, et al. Low-grade serous ovarian cancer: state of the science. Gynecol Oncol. 2020;156(3):715-725.
- Plaxe SC. Epidemiology of low-grade serous ovarian cancer. Am J Obstet Gynecol. 2008;198(4):459.e1-8.
- Banerjee SN, Ring KL, Nieuwenhuysen EV, et al. Initial efficacy and safety results from ENGOT-ov60/GOG-3052/RAMP 201: a phase 2 study of avutometinib (VS-6766) ± defactinib in recurrent low-grade serous ovarian cancer (LGSOC). J Clin Oncol. 2023;41(suppl 16):5515. doi:10.1200/JCO.2023.41.16_suppl.5515
- De Decker K, Wenzel HHB, Bart J, et al. Stage, treatment and survival of low-grade serous ovarian carcinoma in the Netherlands: a nationwide study. Acta Obstet Gynecol Scand. 2023;102(3):246-256.
- Sun CC, Andrews N, Ludemann J, et al. Voices of women with low-grade serous ovarian cancer: results from a multinational survey. Poster #2269 presented at: Society of Gynecologic Oncology Annual Meeting on Women’s Cancer; March 16-18, 2024; San Diego, CA.
- Gershenson DM, Miller A, Brady WE, et al. Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): an international, randomised, open-label, multicentre, phase 2/3 trial. Lancet. 2022;399(10324):541-553.
- American Society of Clinical Oncology (ASCO) Post. GOG 3026: durable responses with ribociclib plus letrozole in low-grade serous ovarian cancer. Accessed July 9, 2024. https://ascopost.com/issues/may-25-2023/gog-3026-durable-responses-with-ribociclib-plus-letrozole-in-low-grade-serous-ovarian-cancer/
- Farley J, Brady WE, Vathipadiekal V, et al. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. Lancet Oncol. 2013;14(2):134-140.
- Monk BJ, Grisham RN, Banerjee S, et al. MILO/ENGOT-ov11: binimetinib versus physician’s choice chemotherapy in recurrent or persistent low-grade serous carcinomas of the ovary, fallopian tube, or primary peritoneum. J Clin Oncol. 2020;38(32):3753-3762.
- Ricciardi E, Baert T, Ataseven B, et al. Low-grade serous ovarian carcinoma. Geburtsh Frauenheilk. 2018;78(10):972-976.
- Femara® [Prescribing Information]. East Hanover, NJ: Novartis Pharmaceuticals; 2014.
- Arimidex® [Prescribing Information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP.; 2010.
- Henry NL, Giles JT, Ang D, et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat. 2008;111(2):365-372.
- Avastin® [Prescribing Information]. San Francisco, CA: Genentech, Inc.; 2009.
- Lazurko C, Linder R, Pulman K, et al. Bevacizumab treatment for low-grade serous ovarian cancer: a systematic review. Curr Oncol. 2023;30(9):8159-8171.
- Grisham RN, Slomovitz BM, Andrews N, et al. Low-grade serous ovarian cancer: expert consensus report on the state of the science. Int J Gynecol Cancer. 2023;33(9):1331-1344.
- Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015;7(2):122-136.
- Donnellan PP, Douglas SL, Cameron DA, et al. Aromatase inhibitors and arthralgia. J Clin Oncol. 2001;19(10):2767.
- Goss PE, Strasser K. Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol. 2001;19(3):881-894.
- Daud A, Tsai K. Management of treatment-related adverse events with agents targeting the MAPK pathway in patients with metastatic melanoma. Oncologist. 2017;22(7):823-833.
- Manousaridis I, Mavridou S, Goerdt S, et al. Cutaneous side effects of inhibitors of the RAS/RAF/MEK/ERK signalling pathway and their management. J Eur Acad Dermatol Venereol. 2013;27(1):11-18.
- Fortes BH, Tailor PD, Dalvin LA. Ocular toxicity of targeted anticancer agents. Drugs. 2021;81(7):771-823.
- Gershenson DM, Cobb LP, Westin SN, et al. Contemporary primary treatment of women with stage II-IV low-grade serous ovarian/peritoneal cancer (LGSOC): determinants of relapse and disease-free survival. Gynecol Oncol. 2022;167(2):139-145.
- Grabowski JP, Harter P, Heitz F, et al. Operability and chemotherapy responsiveness in advanced low-grade serous ovarian cancer. An analysis of the AGO Study Group metadatabase. Gynecol Oncol. 2016;140(3):457-462.
- Giannecchini GV, da Silva JL, de Oliveira Bretas G, et al. Exploring novel approaches in the systemic therapy of low-grade serous carcinoma of the ovary: a literature review. Front Med (Lausanne). 2024;11:1366603.
- Nasioudis D, Wang X, Dhillon G, et al. Impact of adjuvant chemotherapy on the overall survival of patients with advanced-stage low-grade serous ovarian carcinoma following primary cytoreductive surgery. Int J Gynecol Cancer. 2023;33(12):1906-1912.
- Gershenson DM, Bodurka DC, Coleman RL, et al. Hormonal maintenance therapy for women with low-grade serous cancer of the ovary or peritoneum. J Clin Oncol. 2017;35(10):1103-1111.
- Younus J, Kligman L. Management of aromatase inhibitor-induced arthralgia. Curr Oncol. 2010;17(1):87-90.
- Crew KD, Greenlee H, Capodice J, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25(25):3877-3883.
- Gupta A, Henry NL, Loprinzi CL. Management of aromatase inhibitor-induced musculoskeletal symptoms. JCO Oncol Pract. 2020;16(11):733-739.
- Crew KD, Capodice JL, Greenlee H, et al. Randomized, blinded, sham-controlled trial of acupuncture for the management of aromatase inhibitor-associated joint symptoms in women with early-stage breast cancer. J Clin Oncol. 2010;28(7):1154-1160.
- Irwin ML, Cartmel B, Gross CP, et al. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol. 2015;33(10):1104-1111.
- Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129(1-2):210-223.
- Henry NL, Unger JM, Till C, et al. Association between body mass index and response to duloxetine for aromatase inhibitor-associated musculoskeletal symptoms in SWOG S1202. Cancer. 2019;125(12):2123-2129.
- Hadji P, Aapro MS, Body JJ, et al. Management of aromatase inhibitor-associated bone loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol. 2017;7:1-12.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer Version 3.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed July 17, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org
- Crane EK, Sun CC, Ramirez PT, et al. The role of secondary cytoreduction in low-grade serous ovarian cancer or peritoneal cancer. Gynecol Oncol. 2015;136(1):25-29.
- Manning-Geist B, Gordhandas S, Liu YL, et al. MAPK pathway genetic alterations are associated with prolonged overall survival in low-grade serous ovarian carcinoma. Clin Cancer Res. 2022;28(20):4456-4465.
- Grisham RN, Manning-Geist BL, Chui MH. The highs and lows of serous ovarian cancer. Cancer. 2023;129(17):2613-2620.