Innovative approaches under investigation
There is a need for more treatment options for patients diagnosed with LGSOC2
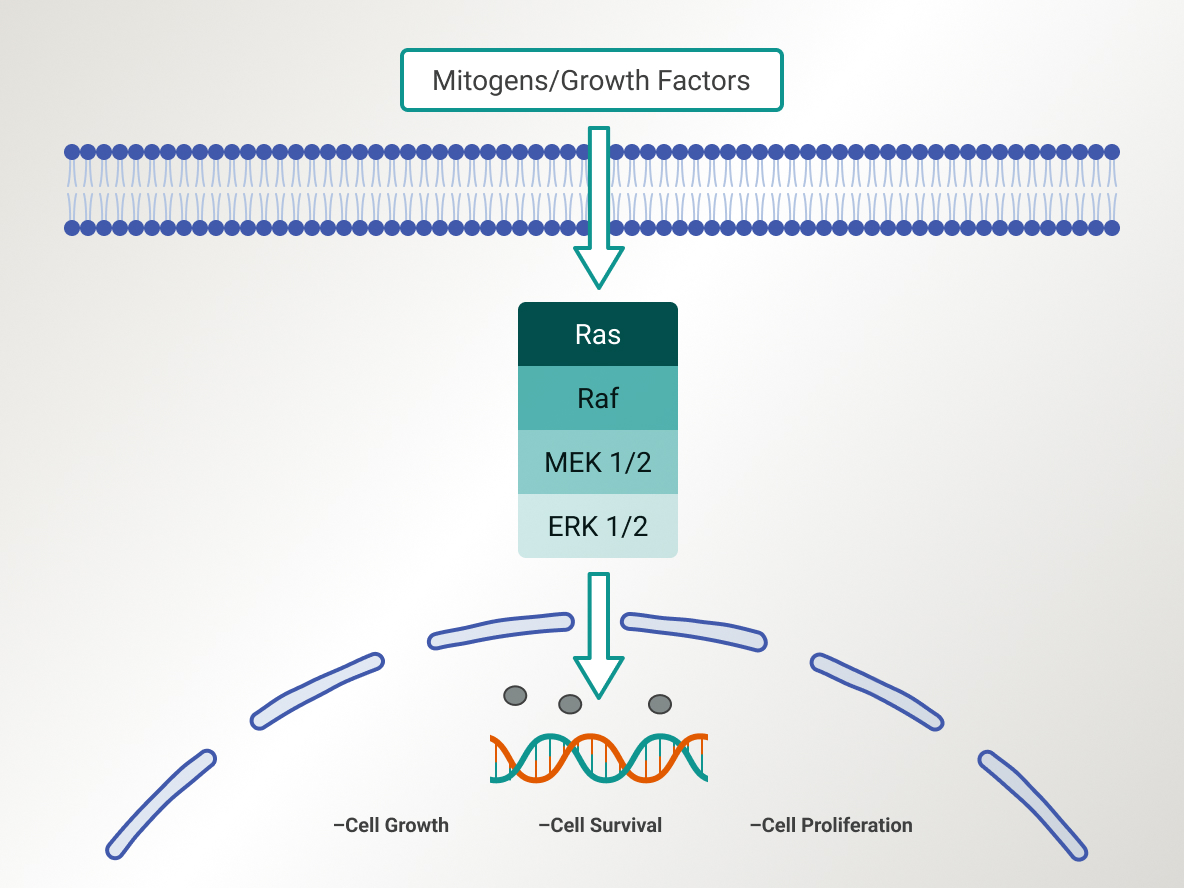
RAS/MAPK pathway
A key to improving outcomes
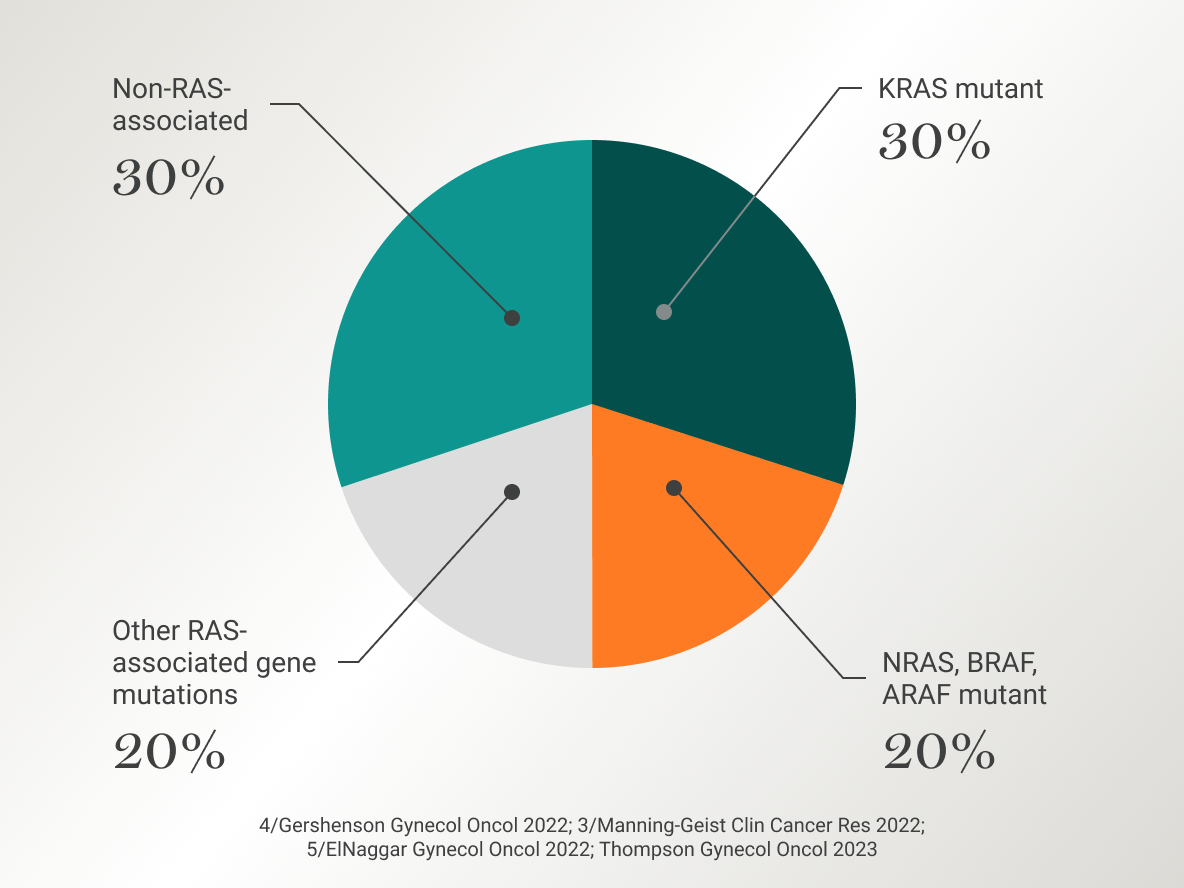
Alterations in the RAS/MAPK pathway, which include genes such as KRAS, BRAF, NRAS, and EIF1AX, are prominent in LGSOC.3
These alterations are uniquely observed in ~60% of LGSOC tumors.3
The mitogen-activated protein kinase (RAS/MAPK) pathway plays a crucial role in controlling gene expression, cellular growth, and survival mechanisms. Anomalies in the RAS/MAPK pathway have been strongly associated with the onset and advancement of various cancer forms, including LGSOC. The strategy of targeting and inhibiting the RAS/MAPK pathway is at the forefront of current research efforts. Despite this, the observed efficacy has been restricted, underscoring the continued need for dedicated research in this area.3,4
Simplified schematic of the MAPK pathway1
A key signaling pathway that regulates a wide variety of cellular processes including proliferation, apoptosis, and stress responses, the MAPK pathway plays a crucial role in LGSOC and serves as an important target for therapies such as MEK and RAF inhibitors.1
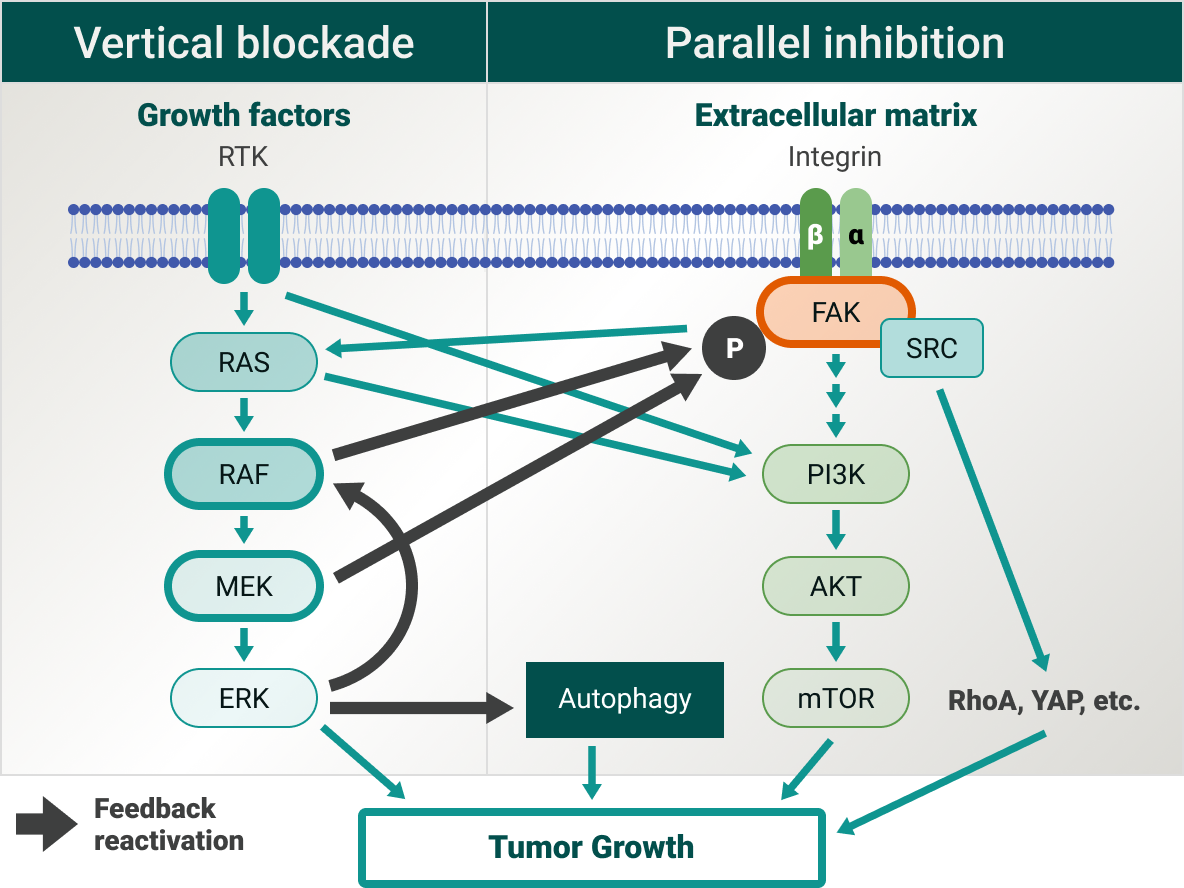
Overcoming the challenge of multiple resistance mechanisms
RAS is one of the best-known signaling proteins implicated in the initiation and progression of cancer. While the RAS/MAPK pathway is promising, it has been observed that blocking any single node of the RAS/MAPK pathway can cause reactivation of another node of the pathway or activation of parallel pathways as a resistance mechanism.8
Single-target therapies (eg, MEK-only inhibitors) are associated with resistance and may not be the best avenue to slowing tumor growth, and currently finding tolerable combination regimens with MEK-only inhibitors has been challenging.9 In addition, blocking the RAS/MAPK pathway can be circumvented through parallel pathways/signaling targets such as PI3K/AKT/mTOR,9 FAK,10 RhoA,12 and YAP13
FAK is at the intersection of various signaling pathways promoting cancer growth and metastasis,12 and there are currently over 15 clinical trials to better understand the FAK pathway and the implications in multiple cancer types.
Ongoing studies of therapies targeting multiple nodes in the RAS/MAPK pathway may guide future treatment options3
Sign up to receive the latest LGSOC
consensus publication
Other important targets in LGSOC
In addition to KRAS and BRAF, several other genetic alterations are under investigation for their potential involvement in the pathogenesis of LGSOC. Some of these include CDKN 2A/2B deletion, CDK 4/6, MEK, NRAS, ERBB2, ERBB3, PIK3CA alterations, Neurofibromin 1 (NF1), and chromosome 1p36 deletion.2,3,14,15
Snapshot of emerging treatment options in LGSOC
Updated as of 9/17/2023
| Trial | Intervention | Patient or population | Reference |
| GOG 0239 Phase II13 | Selumetinib MEK inhibitor | Recurrent LGSOC | Farley et al.16 |
| GOG 281 Phase II/III13 | Trametinib MEK ½ inhibitor | Recurrent LGSOC | Gershenson et al.17 |
| MILO Phase III13 | Binimetinib MEK ½ inhibitor | Recurrent LGSOC | Monk et al.18 |
| RAMP-201 Phase II (ongoing)13 | Avutometinib (VS-6766) RAF/MEK clamp + defactinib (VS-6063) FAK inhibitor | Recurrent LGSOC with and without KRAS mutation | Banerjee SN et al.19 |
Ongoing clinical trials in LGSOC14,20,21
Updated as of 9/15/2023
| Drug | MoA | Phase | Trial | Treatment Setting |
| Trametinib + navitoclax | BC12 inhibitor | Ib/II | NCT02079740 | Advanced solid tumors that carry KRAS or NRAS mutations, including ovarian tumors |
| Letrozole | Aromatase inhibitor | III | MATAO NCT04111978 | ER-positive HGSC, LGSOC, or endometrial ovarian tumors, stages II-IV whose cancer has not progressed on platinum-based chemotherapy |
| Letrozole + paclitaxel and carboplatin | Aromatase inhibitor | III | NRG-GY-019 NCT04095364 | Stage II-IV LGSOC, fallopian tube, or primary peritoneal cancer |
| Avutometinib + defactinib | Kinase inhibitors | III | NCT06072781 | Recurrent LGSOC |
| Onapristone ± anastrozole | PR inhibitor | II | NCT03909152 | PR-positive gynecologic cancers including recurrent LGSOC |
| Abemaciclib + fulvestrant | CDK4/6 inhibitor | II | NCT03531645 | Neoadjuvant therapy in women with unresectable, untreated stage III-IV LGSOC |
| Avutometinib + defactinib | Kinase inhibitors | II | NCT04625270 | Recurrent LGSOC with and without a KRAS mutation |
| Ribociclib + letrozole | CDK 4/6 aromatase inhibitor | II | GOG 3026 NCT03673124 | Recurrent LGSOC |
| Regorafenib + fulvestrant | Antiangiogenic agent | II | NCT05113368 | Recurrent LGSOC |
| Pembrolizumab + chemotherapy | ICI | II | PERCEPTION NCT04575961 | Platinum-sensitive recurrent LGSOC |
| Biomarker-driven therapies | Multiple MOAs based on biomarker | II | BOUQUET NCT04931342 | Persistent or recurrent rare epithelial ovarian, fallopian tube, or primary peritoneal tumors |
For a complete list, visit clinicaltrials.gov.
The role of clinical trials in
treatment progress

Unique targets
In recent years, new insights into the clinical and molecular features of LGSOC have led to prospective clinical trials that focus on improving management and treatment of disease.1,2
Targeting unique characteristics of LGSOC and learning from prognostic markers may help tailor sequencing and treatment options.1 Current clinical trials in LGSOC focus on:
- Optimizing front-line strategies in the neoadjuvant and adjuvant settings1
- Maximizing the effectiveness of recurrence strategies with endocrine therapy and RAS/MAPK pathway-targeting drugs1
Trials in progress
Though this is a rare disease, clinically meaningful research has successfully enrolled patients with LGSOC in prospective clinical trials performed by NRG Oncology, ENGOT, and GOG Partners.1 Clinical trials focused on maximizing the effectiveness of endocrine therapy and RAS/MAPK pathway-targeting drugs are likely to shape how this rare disease is treated in the decade to come.1

Receive information about LGSOC and more
- Grisham RN, Manning‐Geist BL, Chui MH. The highs and lows of serous ovarian cancer. Cancer. 2023;1-8. doi:10.1002/cncr.34903
- Zwimpfer TA, Tal O, Geissler F, Coelho R, et al. Low grade serous ovarian cancer – A rare disease with increasing therapeutic options. Cancer Treat Rev. 2023;112:102497. doi:10.1016/j.ctrv.2022.102497
- Manning-Geist B, Gordhandas S, Liu YL, et al. MAPK pathway genetic alterations are associated with prolonged overall survival in low-grade serous ovarian carcinoma. Clin Cancer Res. 2022;28:4456-4465. doi:10.1158/1078-0432.CCR-21-4183
- Gershenson DM, Cobb LP, Westin SN, et al. Contemporary primary treatment of women with stage II-IV low-grade serous ovarian/peritoneal cancer (LGSOC): determinants of relapse and disease-free survival. Gynecol Oncol. 2022;167(2):139-145. doi:10.1016/j.ygyno.2022.09.005
- ElNaggar A, Robins D, Baca Y, et al. Genomic profiling in low grade serous ovarian cancer: Identification of novel markers for disease diagnosis and therapy. Gynecol Oncol. 2022;167(2):306-313. doi:10.1016/j.ygyno.2022.09.022
- Thomson JP, Hollis RL, van Baal J, et al. Whole exome sequencing of low grade serous ovarian carcinoma identifies genomic events associated with clinical outcome. Gynecol Oncol. 2023;174:157-166. doi:/10.1016/j.ygyno.2023.04.011
- Nasioudis D, Latif NA, Ko EM, et al. Next generation sequencing reveals a high prevalence of pathogenic mutations in homologous recombination DNA damage repair genes among patients with uterine sarcoma. Gynecol Oncol. 2023 Oct;177:14-19. doi:10.1016/j.ygyno.2023.07.020. Epub 2023 Aug 21.
- Braicu C, Buse M, Busuioc C, et al. A comprehensive review on MAPK: a promising therapeutic target in cancer. Cancers (Basel). 2019;11(10):1618. doi:10.3390/cancers11101618
- Kun E, Tsang YTM, Ng CW, et al. MEK inhibitor resistance mechanisms and recent developments in combination trials. Cancer Treat Rev. 2021;102137. doi:10.1016/j.ctrv.2020.102137
- Lee BY, Timpson P, Horvath LG, Daly R. FAK signaling in human cancer as a target for therapeutics. Pharmacol Ther. 2015;146:132-149. doi:10.1016/j.pharmathera.2014.10.001
- Soriano O, Alcón-Pérez M, Vicente-Manzanares M, et al. Review: The crossroads between RAS and RHO signaling pathways in cellular transformation, motility and contraction. Genes. 2021;12(6):819.
- Lin L, Sabnis AJ, Chan E, et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nature. 2015;47(3):250-256. doi:10.1038/ng.3218
- Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14(9):598-610. doi:10.1038/nrc3792
- Grisham RN, Slomovitz BM, Andrews N, et al. Low-grade serous ovarian cancer: expert consensus report on the state of the science. Published online August 17, 2023. Int J Gynecol Cancer. 2023;33(9):1331-1344. doi:10.1136/ijgc-2023-00460
- Van Nieuwenhuysen E, Busschaert P, Laenen A, et al. Loss of 1p36.33 frequent in low-grade ovarian cancer. Neoplasia. 2019;21(6):582-590. doi:10.1016/j.neo.2019.03.014
- Farley J, Brady WE, Vathipadiekal V, et al. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. Lancet Oncol. 2013;14(2):134-140. doi:10.1016/S1470-2045(12)70572-7
- Gershenson DM. Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): an international, randomised, open-label, multicentre, phase 2/3 trial. Lancet. 2022;399(10324):541-553. doi:10.1016/S0140-6736(21)02175-9
- Monk JB. MILO/ENGOT-ov11: binimetinib versus physician’s choice chemotherapy in recurrent or persistent low-grade serous carcinomas of the ovary, fallopian tube, or primary peritoneum. J Clin Oncol. 2020;38(32):3753-3762. doi:10.1200/JCO.20.01164
- Banerjee SN, Ring KL, Nieuwenhuysen EV, et al. Initial efficacy and safety results from ENGOT-ov60/GOG-3052/RAMP 201: A phase 2 study of avutometinib (VS-6766) ± defactinib in recurrent low-grade serous ovarian cancer (LGSOC). J Clin Oncol. 41. No 16_suppl (June 1, 2023) 5515-5515.
- Basket study of the oral progesterone antagonist onapristone ER (Apristor), alone or in combination with anastrozole in women with progesterone receptor positive (PR+) recurrent granulosa cell tumor, low grade serous ovarian cancer or endometrioid endometrial cancer. ClinicalTrials.gov identifier: NCT03909152. Updated May 3, 2023. Accessed October 16, 2023. https://classic.clinicaltrials.gov/ct2/show/study/NCT03909152
- A phase 3, randomized, open-label study of combination therapy with avutometinib plus defactinib versus investigator’s choice of treatment in patients with recurrent low-grade serous ovarian cancer (LGSOC) (RAMP 301). ClinicalTrials.gov identifier: NCT06072781. Updated October 16, 2023. Accessed October 16, 2023. https://classic.clinicaltrials.gov/ct2/show/study/NCT06072781